pharmaceutical excipients Sulfobutyl Ether-B-cyclodextrin SBECD cas 182410-00-0
|
Betadex Sulfobutyl Ether Sodium |
||
|
Cas no. |
182410-00-0 |
|
|
abbreviation |
||
|
English name |
||
|
Molecular formula |
C42H70-nO35 (C4H8O3S Na)n |
|
|
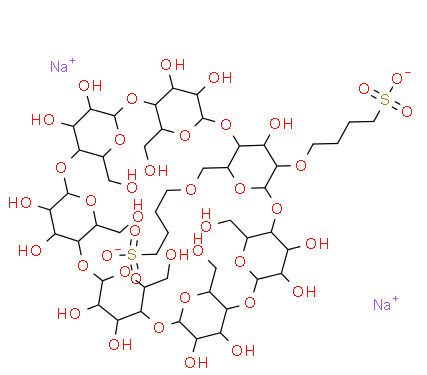
Molecular structure |
||
|
Product qualification |
Product specifications: injection |
|
|
quality standard: USP/EP |
||
|
CDE platform – drug excipient registration number:F20190000285 |
||
|
USA DMF Registration No.:030167 |
||
|
Structure and composition |
The sodium sulfobutylbetacyclodextrin produced by us is β- The product of substitution reaction between cyclodextrin and 1,4-butanesulfolactone. Betacyclodextrin glucose unit is formed by connecting 7-pyranoglucose through a-1 and 4-glycosidic bonds, and the substitution reaction takes place in β- At the 2,3,6 carbon hydroxyl position of the CD glucose unit, the obtained product is SBECD with a degree of substitution of 6.2-6.9. |
|
|
Product features |
It can be used as solubilizer, wetting agent, chelating agent (complexing agent) and multivalent masking agent. Sulfobutylbetacyclodextrin sodium is a new type of anionic highly water-soluble cyclodextrin derivative, which can be well combined with drug molecules to form a non covalent complex, thus improving the stability, water solubility and safety of the drug, reducing renal toxicity, easing drug hemolysis, controlling drug release rate, covering bad smell, etc. It has good solubilization, convenient administration, safety and stability. Betacyclodextrin( β- CD). In contrast, sulfobutylbetacyclodextrin sodium has good water solubility, small hemolysis and low nephrotoxicity, so it is a new pharmaceutical excipient with very broad application prospects. |
|
|
Product performance |
1. Solubility: Neutral, positive and negative APIs can effectively combine with sulfobutylbetacyclodextrin, which can increase the solubility of compounds in the API water by 10 to 25000 times. 2. Convenient administration: Sulfobutylbetacyclodextrin has good biocompatibility and can be administered by injection, oral administration, eye, nose, external use and inhalation. 3. Good safety: Generally, it can be eliminated from the kidney rapidly and completely after administration. In vitro and in vivo acute, metastable and chronic toxicity studies provide safe data and are approved for human drug preparations. 4. Good stability: The interaction with sulfobutylbetacyclodextrin can provide a beneficial protection environment for the API in its lipophilic cavity, while the water-resistant surface provides excellent water solubility and stability. |
|
|
Case application |
Sulfobutylbetacyclodextrin sodium is commonly used in insoluble compounds, such as voriconazole, capezolomide, ziprasidone, aripiprazole, maropenitan (animal medicine), posaconazole, carbamazepine, mefalen, deloxacin, mebendazole, topiramate, omeprazole, clopidogrel, docetaxel, sofibuvir, ziprasidone mesylate, levo phenylalanine mustard, meloxicam Tetrahydroprogesterone and several other nitrogen-containing APl bases are at different clinical stages. |
|
|
Sulfobutyl currently marketed by FDA- β- Cyclodextrin sodium inclusion complex injection mainly includes: Voriconazole (trade name: Vfend) Ziprasidone (trade name: Geodon) Posaconazole (trade name: Noxafil) |
||
|
Remdesivir for injection has started the phase III clinical study of anti new coronary disease (2019 nCoV) in Beijing Sino Japan Friendship Hospital. Gilead has done a lot of research on the dose and safety of Remdisivir. At the clinical dose, Remdesivir shows good overall safety, except for its impact on liver function. Redcevir freeze-dried preparation also contains the following non active ingredients: water for injection, sulfobutyl- β- Sodium cyclodextrin (SBECD), adjust the pH of the preparation to 3.0-4.0 with HCL and/or NAOH, and then freeze dry. According to the current stability data, the freeze-drying can be kept below 30 ℃ for three years. |
||
|
Specific application in products |
1. Application in injections (1) Function: It can increase the solubility and stability of insoluble drugs by increasing solvents and stabilizers, so that insoluble drugs can be developed into injections. (2) Example: The solubility of voriconazole, posaconazole, delafloxacin, docetaxel, ibuprofen and indomethacin was improved by sulfobutylbetacyclodextrin sodium; The stability of carmustine was improved by sulfobutylbetacyclodextrin. |
|
|
2. Application in oral preparations (1) Function: It can increase the solvent and stabilizer, and improve the bioavailability of insoluble drugs. (2) Example: The bioavailability of flunarizine, danazol, hydrogenated prednisolone and prasugrel was improved with sulfobutylbetacyclodextrin. 3. Application in ophthalmic preparations (1) Function: increase solvent and stabilizer, reduce drug irritation. (2) Example: The irritation and stability of pilocarpine, dipivoline, balofloxacin and ganciclovir were improved with sodium sulfonated J-betacyclodextrin. |
||
|
4. Application in nasal preparations (1) Functions: increase the permeability of nasal mucosa, improve the solubility and stability of drugs, and improve the metabolic rate of drugs targeted for administration. (2) Example: Sulfobutylbetacyclodextrin is used to improve the solubility and stability of midazoline. 5. Ointment (1) Function: Improve the solubility and stability of the drug. (2) Example: Sulfobutylbetacyclodextrin is used to improve the solubility and stability of nimesulide. |
||
|
Our advantages |
(1) Quality standard: injection grade, meeting USP/EP requirements. (2) The quality is guaranteed, and has been audited by domestic and foreign customers for many times and has been praised. (3) It has complete qualifications and has obtained the “approval document for the production of pharmaceutical excipients” and DMF filing number. (4) Strong supply capacity, with a capacity of 500 tons/year. (5) Professional domestic and foreign trade personnel, familiar with the operation process. (6) We have a long-term cooperation in professional logistics and express delivery to ensure safe transportation and timely delivery. (7) Provide technical support. |
|
|
Manufacturer advantages |
(1) The manufacturer has a high standard GMP workshop. Strong supply capacity, reliable quality, advanced technology and stable products. (2) The manufacturer has complete production and inspection facilities and has successfully passed the audit of many domestic and foreign pharmaceutical enterprises. People oriented, continuously improve the quality level, and fully meet customer requirements. At present, it has passed ISO9001 quality system certification, ISO14001:2015 environmental management system certification, and IS045001:201 8 occupational health and safety management system certification, thus standardizing the whole process of cyclodextrin production. There are standard processes for product quality from raw material procurement, inspection to the entire production process and warehousing. The product standards comply with USP/EP/CP/enterprise standards. The manufacturer operates in strict accordance with IS09001: 9001 quality system. Conduct quality management and supervision for each production link. Each process of our production is carried out in strict accordance with the procedures specified in our quality manual, and quality problem analysis meetings are held regularly to solve the quality problems encountered in the production process. Strive to provide each customer with qualified products of internal quality. (3) Technical advantages: The manufacturer has established a high standard R&D laboratory, a quality control laboratory, a microbiological laboratory and a synthesis laboratory, and has established a cyclodextrin application R&D center with universities. Manufacturers continue to increase investment in scientific research and technology, strengthen the construction of manufacturers’ R&D strength, expand the R&D team, purchase advanced R&D testing equipment, improve the overall technical strength of manufacturers, and provide a solid guarantee for the stability of product quality. |
|
|
Packing |
(1) Packaging: sterile PE bag+aluminum foil bag (2) Outer package: fiber drum, carton (3) Package specification: Injection grade: 10kg/1 drum, 20kg/1 drum |
|
|
Package size (cm) |
37*37*55 |
|
|
CBM (m3) |
0.075 |
|
|
N.W.(KGS) |
20kg/fiber drum |
|
|
G.W.(KGS) |
22.9kg/fiber drum |
|
|
Pallet Size |
120*120*15 |
|
|
Pallet N.W.(KGS) |
20kgs |
|
|
QTY |
9 fiber drums/layer total 18 fiber drums/pallet |
|
|
Storage conditions |
Storage conditions: sealed and stored at room temperature Validity: 2 years |
|
|
Product specifications |
Contact us for product inspection specifications. Contacts: Bruce Mu/Bruce_mu@labeyond.com |
|